About Us
We are interested in the understanding at a very fundamental level of the structure-properties relationship of complex food systems composed by biopolymers, proteins, biocolloids and surfactants, as well as in the study of their synthetic counterparts, which are used as model reference systems. We study the self-assembly and equilibrium properties of these materials at multiple length scales, in the dilute, semi-dilute and concentrated regime from a soft condensed matter physics perspective. To study the physics regulating the self-organization of these complex fluids, we rely on experimental techniques such as atomic force and transmission electron microscopy, static and dynamic light scattering, as well as small angle x-rays and neutron scattering. We also collaborate with theoretical groups worldwide in order to unravel the self-assembly processes via modern thermodynamic theories.
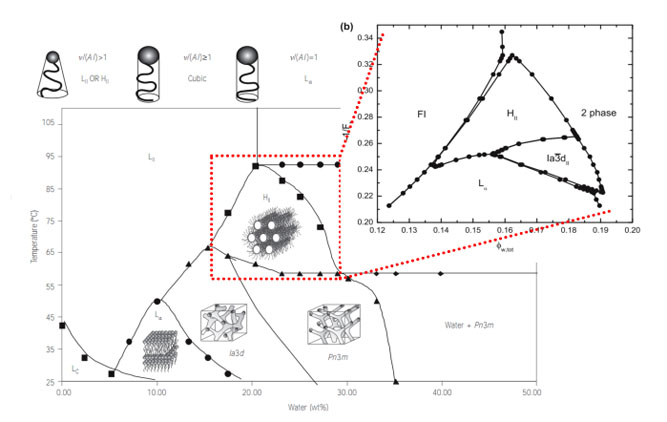
Experimental phase diagram of self-assembled monoglyceride-water lyotropic liquid crystals (from external page Nature Materials, 4, 729 (2005))
Theoretical phase diagram of monoglyceride-water lyotropic liquid crystals as modeled by self-consistent field theory (from external page Phys. Rev. Lett. 99, 187801 (2007))
Amyloid Fibrils Formation, Self-assembly and Physical Properties
For a Journey into Protein Fibrils from Fundamentals to Applications see the Inaugural Lecture
Aggregation of proteins is central to many aspects of daily life, ranging from food technology and pharmaceutical science, to blood coagulation and health disorders, such as sickle-cell disease, arterial thrombosis, or eye cataract formation. In particular, association of proteins into amyloid fibrils is a highly specific process occurring both in-vivo, such as in the Alzheimer, Parkinson or prion-related neurodegenerative diseases, and in-vitro, as in the case of processed food proteins.
Recently, however, protein fibrils are emerging as unique building blocks to assist the design of functional materials in a wide range of contexts and applications. The possibility to efficiently mix them with other components, more traditionally used in the design of high-performance materials, enables new opportunities in materials science and nanotechnology.
We are interested in understanding the association processes converting globular proteins into amyloid fibrils, and how the resulting fibrils assemble in 3D and 2D. To this end we use colloidal and polymer physics concepts, coupled to statistical analysis of microscopy images or scattering experiments.
We are also interested in designing new classes of hybrid nanocomposites in which the protein fibrils serve not only as unique building blocks, but also provide outstanding functionalities to the final hybrid materials. The resulting materials can serve fields as diverse as food science, biomaterials, biosensors and optoelectronics, simply by changing the constituent building blocks.
Lyotropic Liquid Crystals: from Fundamental to Applications
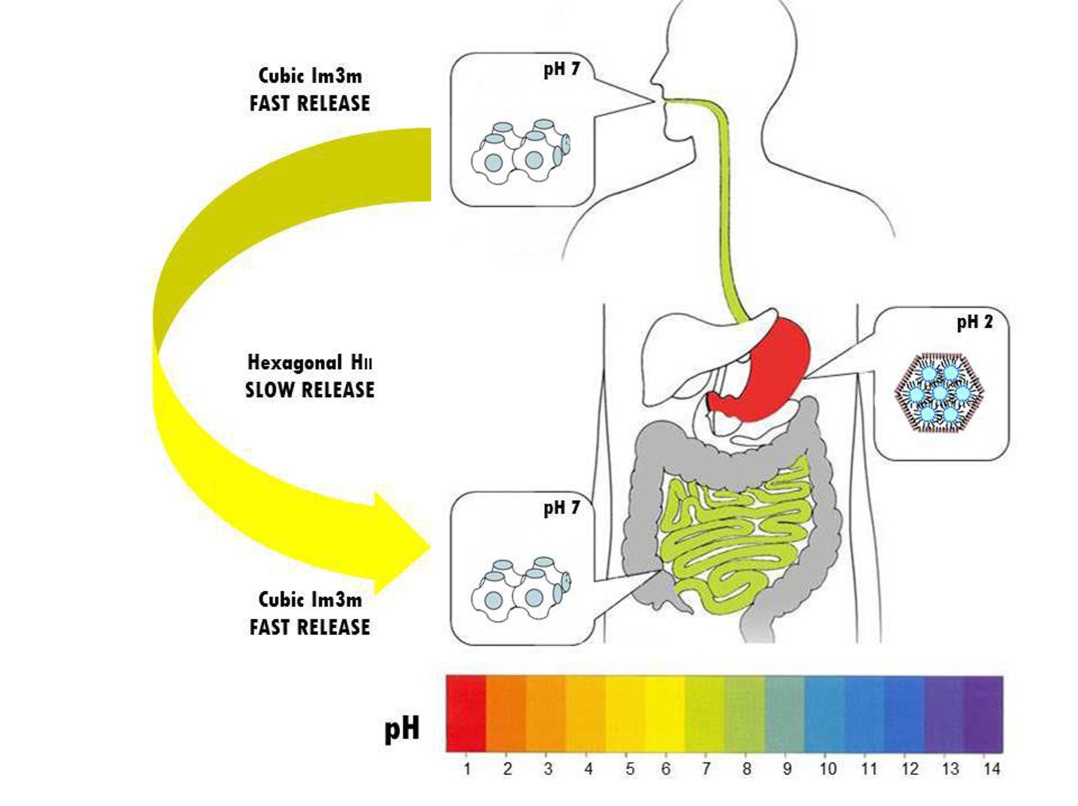
Lipid-based reversed liquid crystalline mesophases, such as bicontinuous cubic, reversed hexagonal or reversed micellar cubic phases, have attracted deep interest in the last few decades due to their potential applications in the food, cosmetic and pharmaceutical arenas. One possible application regarded as very promising is that of controlled delivery of functional ingredients. Different crystallographic structures of the lipid mesophase give access to different diffusion coefficients and distinct diffusion modes. It becomes thus crucial to engineer the space group of the mesophase in a controlled way, and ideally, in a stimuli-responsive manner. We are interested in unraveling the self-assembly of lipids in water into lyotropic mesophases from a very fundamental viewpoint, for which we typically rely on a soft condensed matter approach, borrowing theoretical tools such as self-consistent field theory, developed for block copolymers self-assembly, and experimental characterization based on small angle neutron and x-rays scattering. We also exploit endogenous and exogenous stimuli to control the diffusion in lipid-based mesophases to tune the controlled delivery of molecules and colloids through these systems.
Understanding the single molecule physics of natural polysaccharides
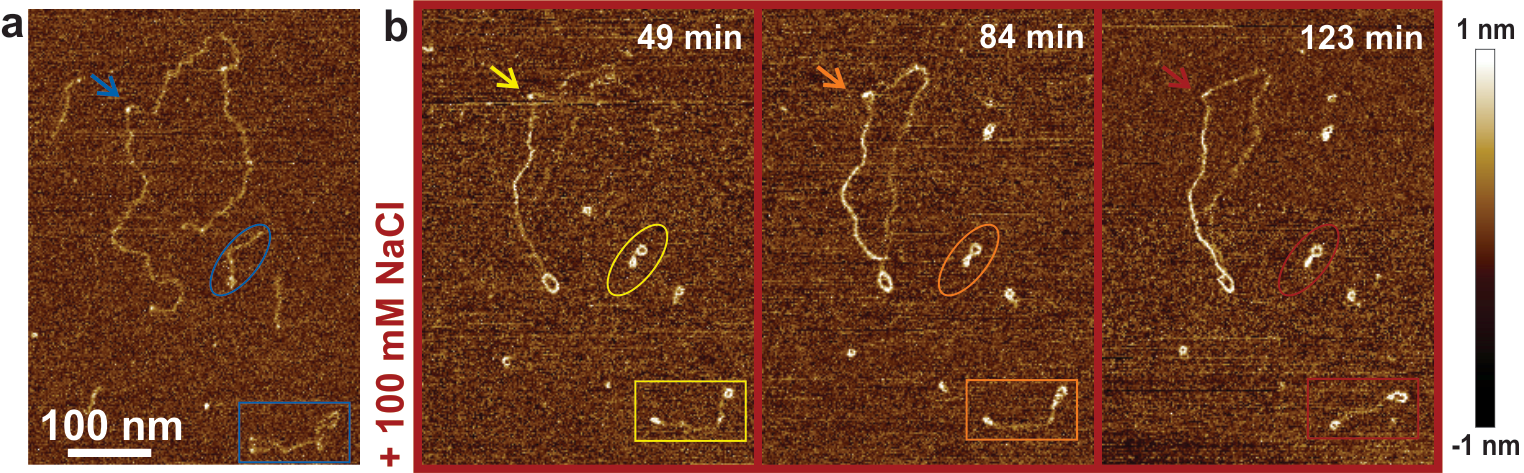
Single molecule imaging techniques such as Atomic Force Microscopy allows a high resolution imaging of biopolymers and biological macromolecules at varying environmental conditions. We are particularly interested in understanding how the structural conformations of polysaccharides with secondary structure such as the anionic carrageenans, develop in the presence of salts close to physiological conditions. These systems are of particular interest due to specific interactions with salt ions, for which traditional polyelectrolytes theories break down. Polymer statistical analysis tools are used to analyze high resolution AFM images and to unravel changes in molecular conformations from random coil the ordered helical conformations. This analysis can shed light also on the nature of the ordered state, that is, it is possible to discriminate whether alpha helical structures exist as single or double helixes.